
Top Performing Drug – Cosentyx (November Edition)
Shots:
-
In continuation of our previous series on the Top-Performing Drug of the month, based on 2021 revenue, this month we have selected Cosentyx and prepared a curated analysis report for our readers
-
Cosentyx is indicated for the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis. It is a human IgG1κ mAb which targets interleukin (IL)-17A
-
PharmaShots presents a concise take on the key features of Cosentyx with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: Secukinumab
Dosage Forms & Strengths
-
Sensoready pen (150 mg/mL)
-
Prefilled syringes (150 mg/mL, 75 mg/0.5 mL)
-
Single-dose vial (150 mg)
Mechanism of Action: Interleukin inhibitor
Originator: Novartis
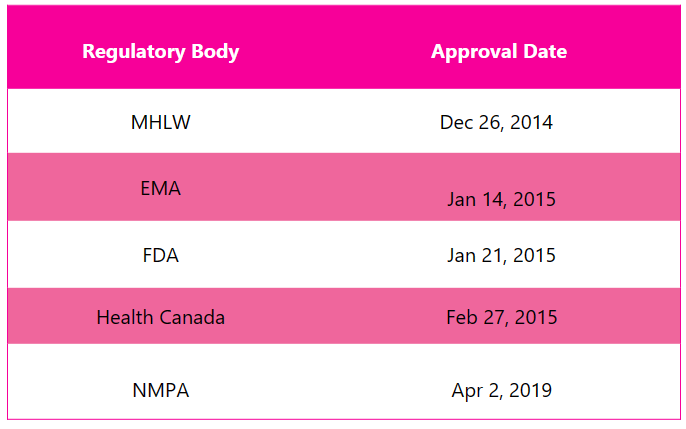
First approvals: The table below depicts the first approvals of Cosentyx from different regulatory agencies.
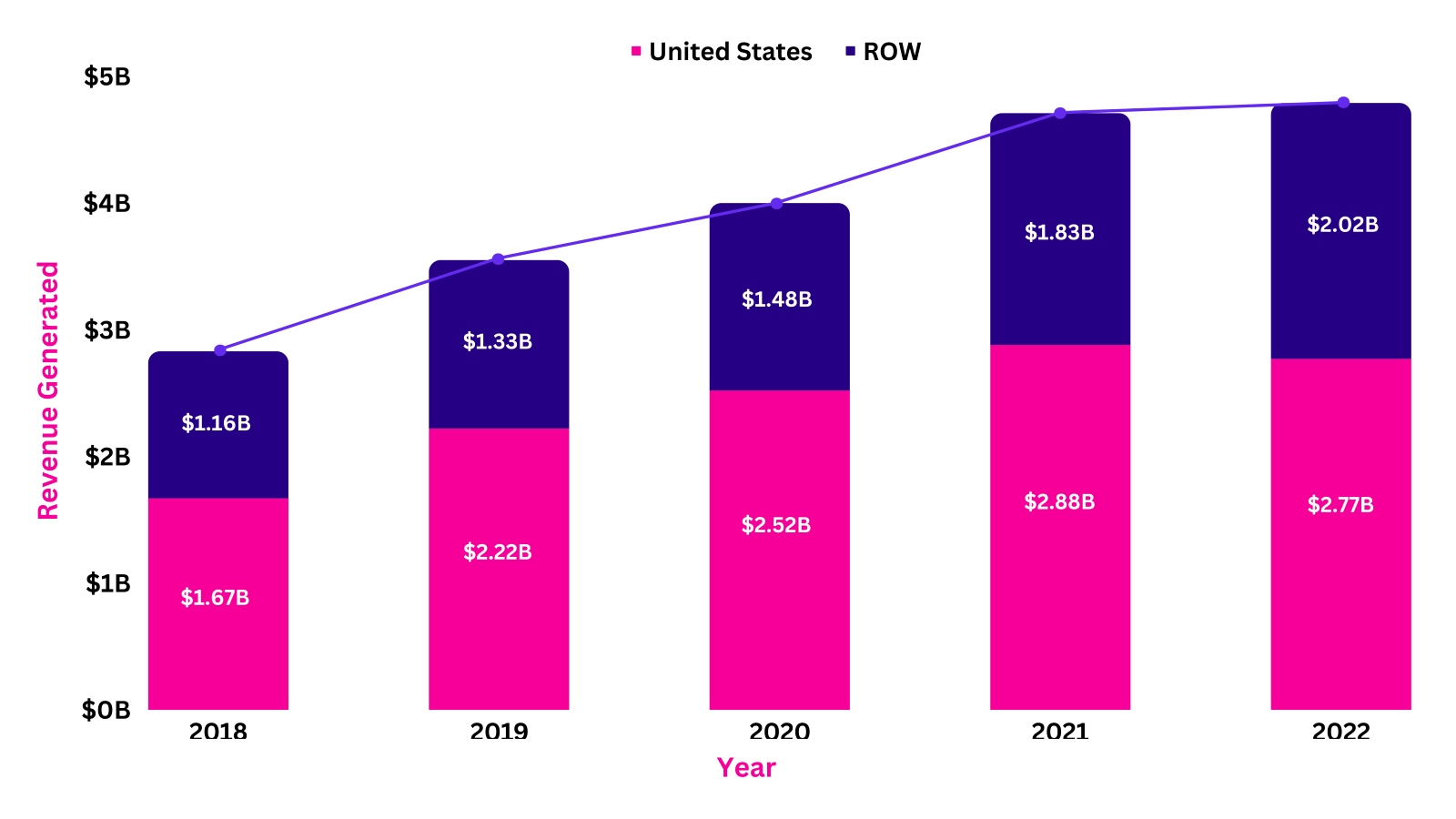
Revenue Analysis5
Over the past five years, the total sales of Cosentyx have substantially grown to generate a high number of profits for Novartis. The highest percentage change in the product’s overall sales was seen in the year 2019 vs 2018, the sales of Cosentyx grew by 25.16% in 2019. This massive increase in the product’s overall sales was attributed to its continued momentum globally led by a strong demand across indications and regions. Moreover, Cosentyx’s approval by the NMPA in March 2019 also played a significant role in the growing sales over the year. Recently, in the year 2022, the sales generated by Cosentyx were $4.79B with $2.77B generated from the US markets and $2.02B from Rest of the World (ROW).
The following graph illustrates the revenue analysis for the last five years' sales of Cosentyx.
Approved Indications6
Cosentyx is a human interleukin-17A antagonist indicated for the treatment of:
-
moderate to severe plaque psoriasis in patients 6 years of age and older who are candidates for systemic therapy or phototherapy
-
active psoriatic arthritis (PsA) in patients 2 years of age and older
-
adults with active ankylosing spondylitis (AS)
-
adults with active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation
-
active enthesitis-related arthritis (ERA) in pediatric patients 4 years of age and older
-
moderate to severe hidradenitis suppurativa (HS) in adults
Clinical Trials Analysis7
Clinical trial analysis is vital for advancing medical science, improving patient care, and making informed decisions about the safety and efficacy of new treatments. It underpins the foundation of evidence-based medicine and regulatory approval processes, leading to better healthcare outcomes and the development of innovative therapies.
The following dashboard illustrates the clinical trials associated with Cosentyx.
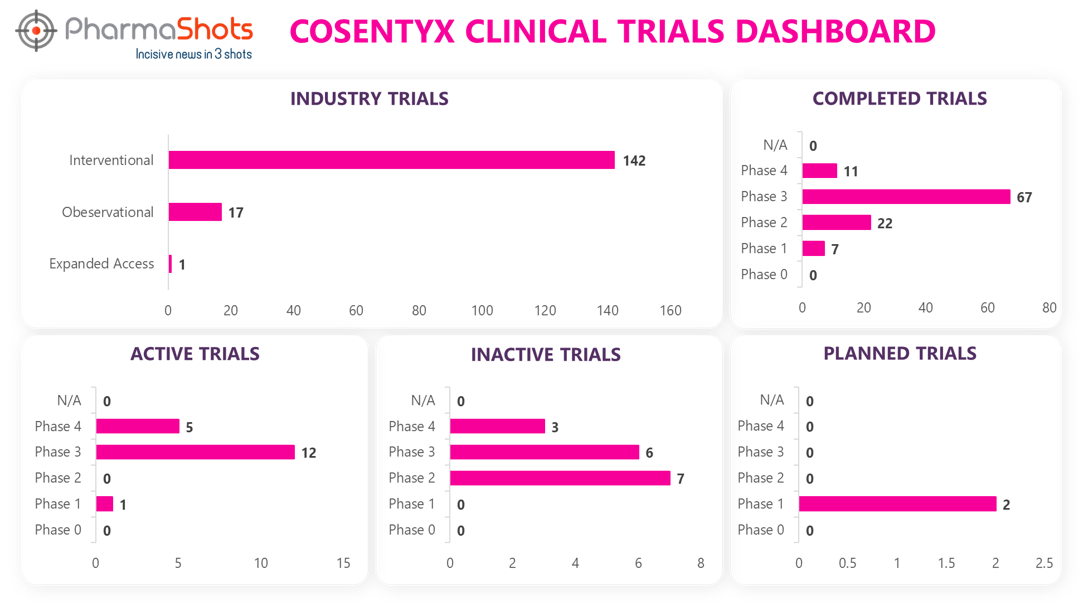
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Cosentyx Trials Representation (Country-wise)7
Below graphs depict ongoing trials investigating Cosentyx.
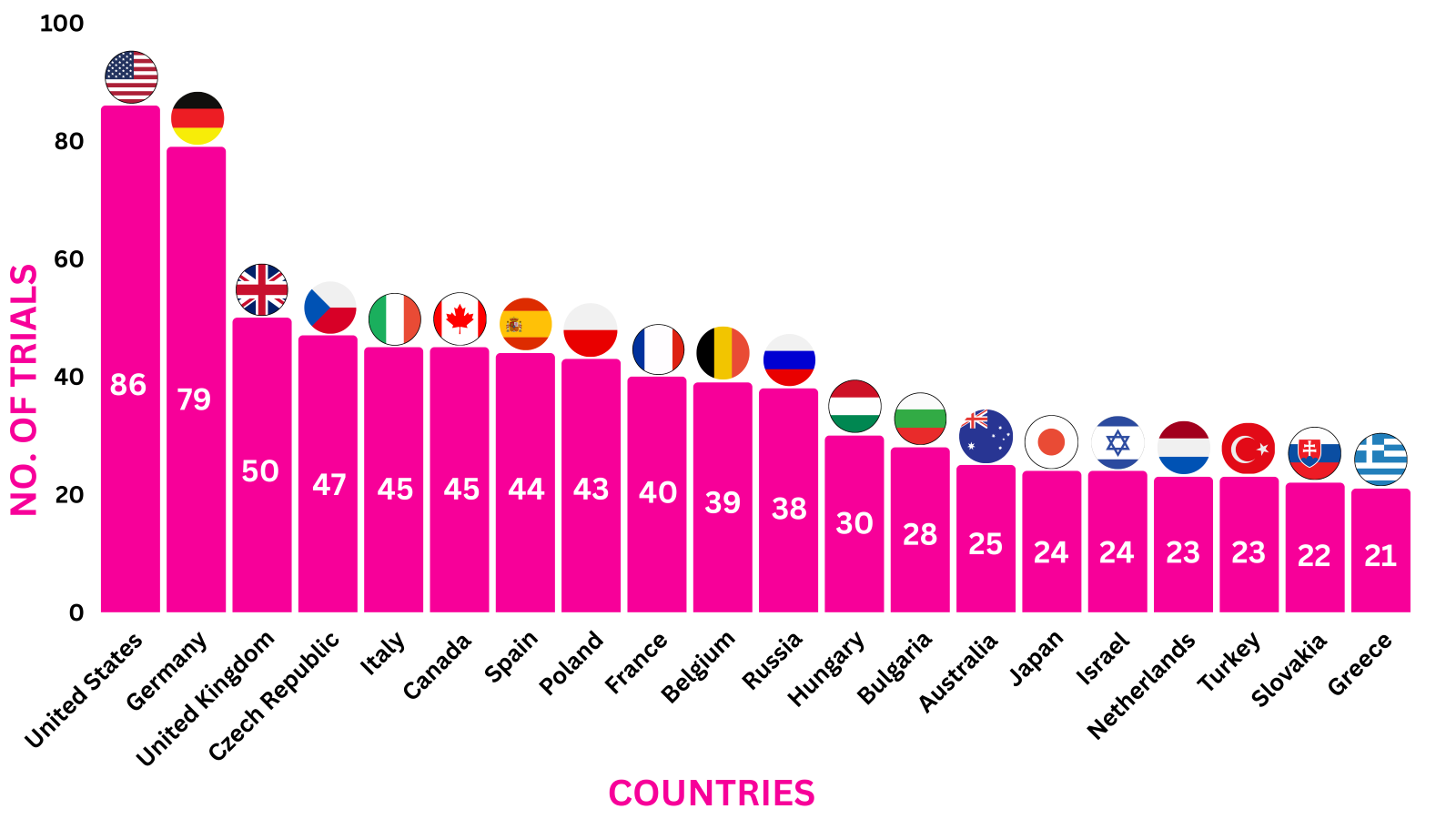
*The data represents trials till Nov 21, 2023
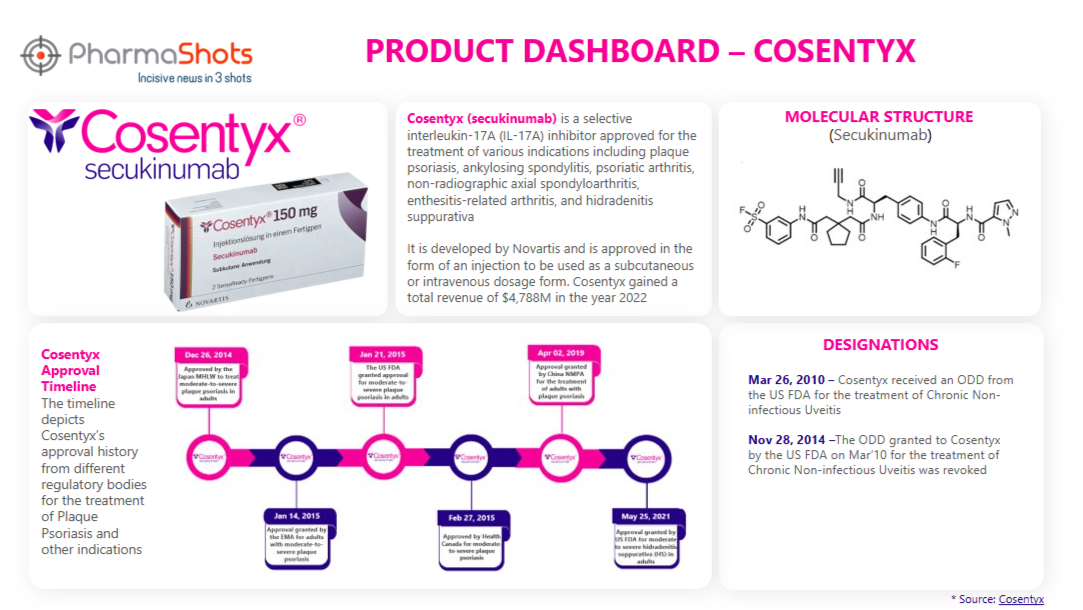
Product Dashboard
PharmaShots presents an illustrative infographic, highlighting essential metrics and pertinent information about Cosentyx.
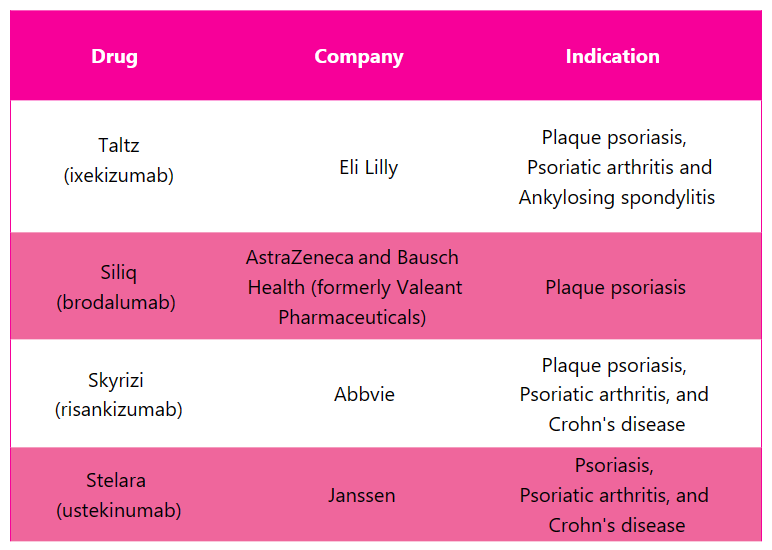
Cosentyx Alternative Drugs3, 6, 10
In response to Cosentyx, several alternative drugs are available in the market to treat different indications. Some of the substitute drug for Cosentyx include:
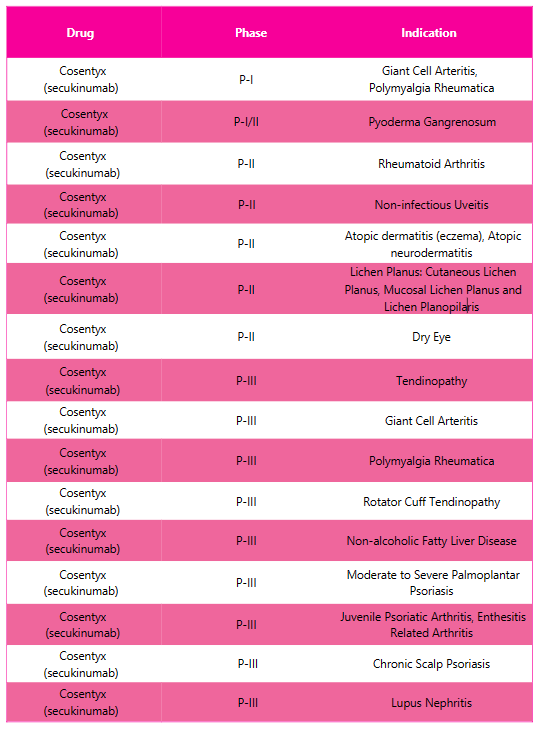
Cosentyx Pipeline Analysis7
PharmaShots presents an extensive analysis of Cosentyx’ pipeline, including the ongoing P-I, P-I/II, P-II and P-III studies for various indications. The table below depicts an overview of these studies.
Cosentyx SWOT Analysis
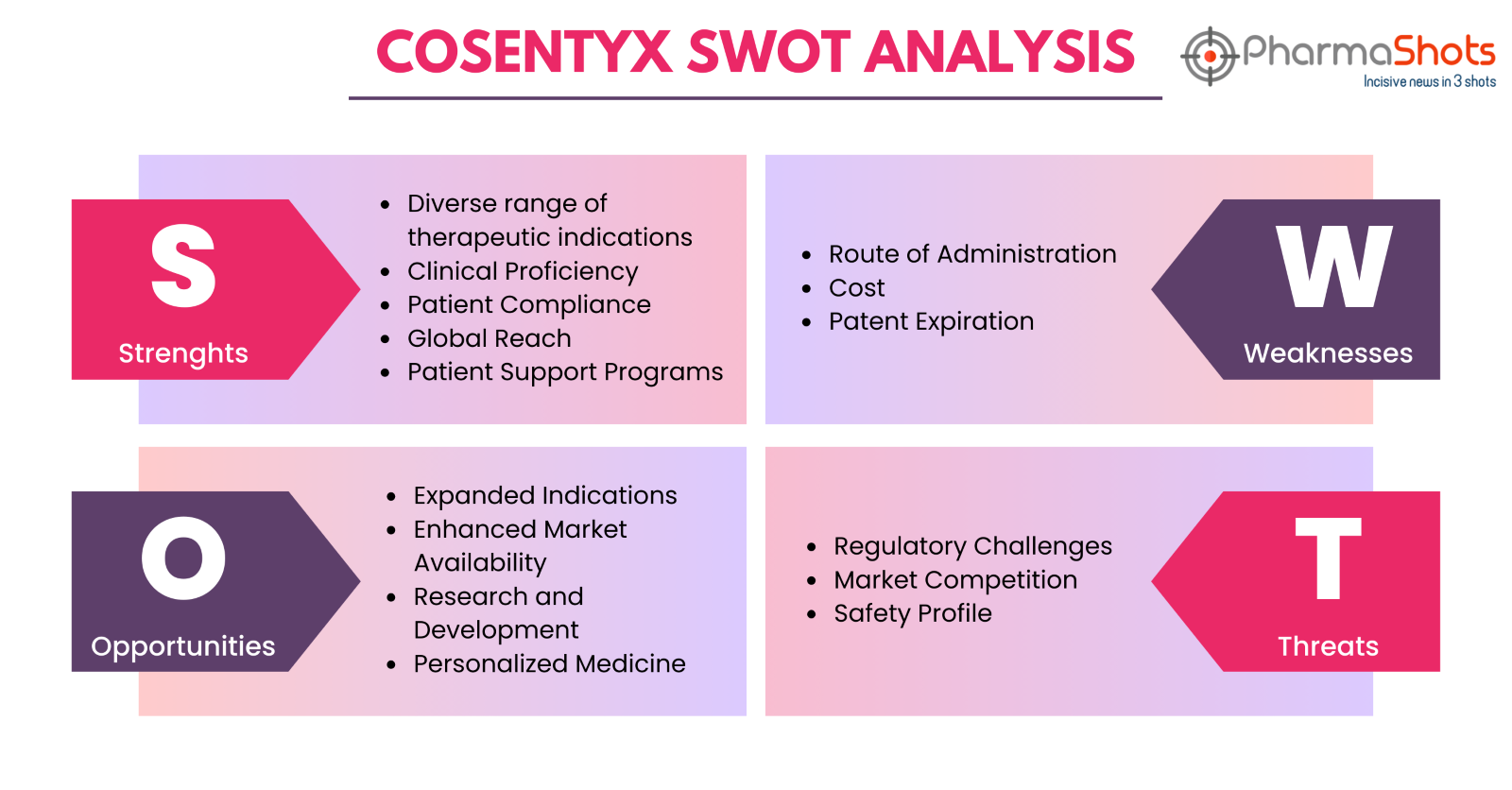
Strengths
-
Diverse range of therapeutic indications: Cosentyx is approved for a wide range of indications, including Plaque Psoriasis (PsO), Psoriatic Arthritis (PsA), Active Ankylosing Spondylitis (AS), Non-radiographic Axial Spondyloarthritis (nr-axSpA), Enthesitis-related Arthritis (ERA), and Hidradenitis Suppurativa (HS). With approvals across a wide range of indications, Cosentyx addresses the needs of a broad range of patients
-
Clinical Proficiency: Under various clinical studies, the product depicts favorable efficacy and safety results in the treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis thereby leaving most of the patients with clear skin
-
Patient Compliance: Cosentyx, under clinical evaluations, shows a positive outcome in terms of patient experiences. Moreover, it comes in the form of an IV or SC injection, making Cosentyx more patient-compliant than its contemporaries due to its easy, consistent, and controlled dosage administration
-
Global Reach: Novartis is a renowned name in the pharmaceutical business and has secured a prominent position among the Top 20 Biopharma Companies. With a strong global presence and an imposing distribution system, the company can promote and commercialize Cosentyx to a great extent
-
Patient Support Programs: Novartis offers patient support programs to help patients with certain factors related to Cosentyx, including education, affordability, and accessibility. These Initiatives improve patients’ compliance with therapy
Weaknesses
-
Route of Administration: Cosentyx is an injectable drug administered through the Subcutaneous or Intravenous route. This route of administration could be a drawback for patients who may face problems while self-injecting the drugs. Moreover, it is often seen that injectable drugs tend to leave an irritating effect at the site of administration
-
Cost: Like other biologic therapies, the cost of Cosentyx is also high. This limits the product's accessibility to those patients who are unable to pay a great amount to purchase the drug or those with inadequate health insurance. Moreover, healthcare payers also face a problem in making these expensive drugs available to patients.
-
Patent Expiration: It is evident that on patent expiration, Cosentyx could face huge competition in the market due to Biosimilar erosion. On the other hand, not only the Biosimilar market but the company’s competitors developing similar molecules with equal therapeutic effects could also pose a great threat to Cosentyx
Opportunities
-
Expanded Indications: Novartis is currently evaluating Cosentyx in different clinical trials for its use to treat other indications than plaque psoriasis. Expanding the list of approved indications for Cosentyx could potentially enhance the company’s patient base
-
Enhanced Market Availability: As Cosentyx is being assessed under several clinical trials for different indications, it has great potential to gain more approvals for new indications. Adding new indications to the product’s portfolio will allow the company to enhance its market availability
-
Research and Development: With a continued analysis of Cosentyx’s application and a great amount being invested by the company in its research and development, Cosentyx may have a great future and could potentially maintain a competitive edge in the market
-
Personalized Medicine: Improvements by the company in terms of personalized medicine could lead to offering more specialized and tailored treatment plans, which in turn could increase Cosentyx's efficacy for particular patient populations
Threats
-
Regulatory Challenges: The company could face a lot of regulatory setbacks during the process of applying Consentyx for approvals under new indications due to unexpected delays in the process or regulatory reforms
-
Market Competition: With a great advancement in the treatment options available for autoimmune diseases, Cosentyx could face huge competition in the market. Not only are the company’s contemporaries fighting to develop new therapies for autoimmune diseases to surpass the demand of Cosentyx, but its biosimilar competitors are not far behind
-
Safety Profile: The reporting of severe adverse events or safety concerns may lead to regulatory assessments, product recalls, or could also harm the product’s reputation and thereby affect the market acceptance and uptake of Cosentyx
Patient Stories6
Patients' stories are the key resources as they provide a holistic perspective on the impact of medications on individuals' lives, improve healthcare decision-making, and contribute to the overall understanding of the drug's efficacy and safety in real-world settings. We have summarized some of the patients’ stories for Cosentyx:
-
DEWEY’S STORY: Dewey felt the breaking point in his life while dealing with psoriasis, when a cashier lady at a store refused to receive cash from him and asked for credit card because of his skin condition which she thought could be infectious. As a professional wrestler, Dewey is all about wearing less gear and showing off his clear skin and this incident made him feel inferior. After starting on cosentyx he has been clear for over 2 years and loves to show his authentic smile without the need to hide his skin.
-
NADIA’S STORY: Nadia was diagnosed with psoriasis around the age of 16 or 17 years and had to suffer through her life’s precious moments like graduation due to her skin condition. But she always believed that behind her psoriasis, behind her scales and plaque she did have beautiful skin and when she found cosentyx she thought it was made for her. She says “4 years clear. I feel so much better”.
-
MARK’S STORY: Mark has lived with pain since his mid-twenties, it started as lower back pain, didn’t know what it was, and no doctor could tell him how they could fix it. Most of them gave ibuprofen for it. It didn’t do anything. Then his chiro practitioner caught AS and recommended a Rheumatologist. He asked the doctor if cosentyx can be tried for his condition and rest is his success story in his words “I have been living with ankylosing spondylitis for over 20 years, my disease hasn’t cured, but I can hardly tell it's there now. Once every 4 weeks I give myself a shot of Cosentyx, that is just part of my routine. Cosentyx pen is easy, you never see the needle, I don’t feel any pain. Five years later I still move and feel better. Nothing holds me back anymore. I wish they had it 20 years ago when I first felt that pain. I would have started taking it then only.”
KOL* Reviews4, 8, 9
KOL reviews offer valuable perspectives on different products and services. These reviews prove beneficial for consumers who engage in product research and prefer to read multiple reviews before making a purchase. Here are a few KOL reviews regarding Cosentyx
-
Philip J. Mease, Clinical Professor at the University of Washington School of Medicine said, "The approval of Cosentyx as an IV formulation is an important milestone for patients because it expands the treatment options available to them with a different mechanism of action than existing biologic IV therapies, along with the comfort and familiarity of an established treatment."
-
Christos C. Zouboulis, President of the European Hidradenitis Suppurativa Foundation said, “With only one currently approved treatment option, I see HS patients with a tremendous need for alternatives that reduce the disabling physical symptoms of HS, improve the emotional burden and help partially avoid invasive surgery, if treating early. This expanded approval offers physicians an additional effective and, for dermatologists, familiar treatment choice that we can feel confident in prescribing for this complex and challenging disease.”
-
Victor Bultó, President of Novartis US said, “Cosentyx can offer effective, lasting relief from HS symptoms so that people with HS have a chance to live every day with confidence. With this sixth indication approval for Cosentyx – along with ongoing studies in numerous other conditions – we are reaffirming our commitment to reimagine medicine for those living with immunological diseases.”
-
Haseeb Ahmad, President of Novartis EU said, “Since its first approval in 2015, Cosentyx has been used to treat more than 1 million people worldwide. We are pleased to bring Cosentyx as a much needed and trusted treatment option that brings rapid and sustained symptom relief to HS patients. With established market access and patient support programs, Novartis is in a strong position to support fast and widespread access to Cosentyx. This milestone approval is a major step forward in our ambition to deliver quality medicines that alleviate major unmet medical needs.”
-
David Epstein, Division Head at Novartis Pharmaceuticals said, “The results from our studies have shown that the majority of patients treated with Cosentyx have a significant reduction in their signs and symptoms of ankylosing spondylitis and psoriatic arthritis, and show major improvements in their ability to undertake everyday activities.”
* Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
-
PubMed
-
EMA
-
Drugs.com
-
Novartis PR
-
Novartis Annual Report
-
Cosentyx.com
-
ClinicalTrials.gov
-
Globe Newswire
-
PR Newswire
-
Medical News Today
Related Post: Top Performing Drug – Tagrisso (October Edition)
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.













